Malaysia has been a member of The Pharmaceutical Inspection Co-operation Scheme (PIC/S) since 2002. A country that joins PIC/S will recognize GMP inspections and assessments performed by other PIC/S member countries, hence reducing the need for repeated inspections. Costs are reduced and time to market is quicker for products by manufacturers with PIC/S country approved GMP.
As a manufacturer, if your products are approved in PIC/S member countries, it will open up more markets for you worldwide and much quicker.
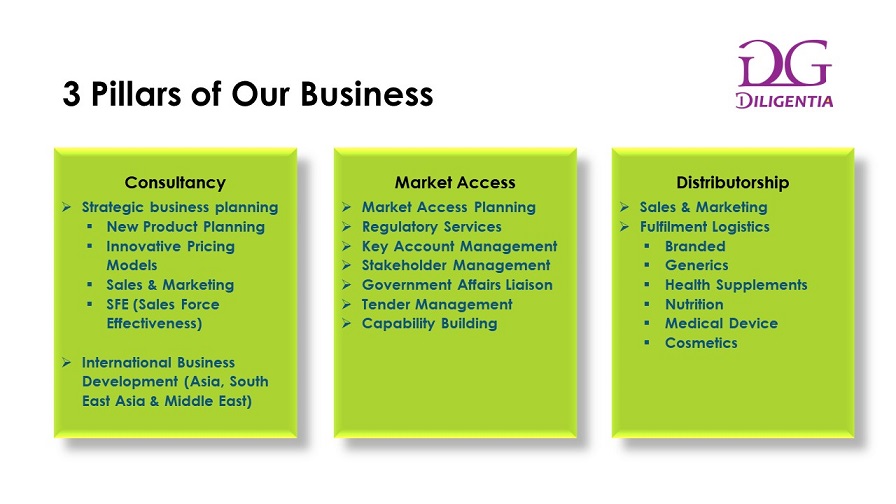
If you are keen to explore further, please reach out to us. We provide exceptional consultancy services and advice to clients and principals. No company is too big or too small as long as you are keen to expand your business, feel free to explore your market needs with us. You’d be surprised as to what we can offer.